PARTNER WITH US
Becoming the Go-To Provider within the Surging mRNA Analytical & Quality Control Communities
As biopharma race to approve and commercially release their novel mRNA therapeutics and vaccines, the analytical development and quality control priorities have never been higher as they navigate the evolving regulatory seas.
However, to enable them to develop safer, efficient and consistent mRNA medicines, they need support in:
Analytical & Assay Development
Screening & Sequencing Technologies
mRNA-Specific Services
Structural Analysis & Cryo-EM
Vaccine
CMO/
CDMO
The 4th mRNA Analytical Development & Quality Control Summit served as a central hub to showcase your expertise and benchmark your brand as a key solutions provider and thought leader, through our bespoke commercial packages.
Interested in Partnering?
I’m Jack, here to help and happy to set up a call so we can discuss your commercial objectives within the mRNA and vaccine space to establish whether partnering with this meeting aligns with your strategy.

Who Could You Have Met?
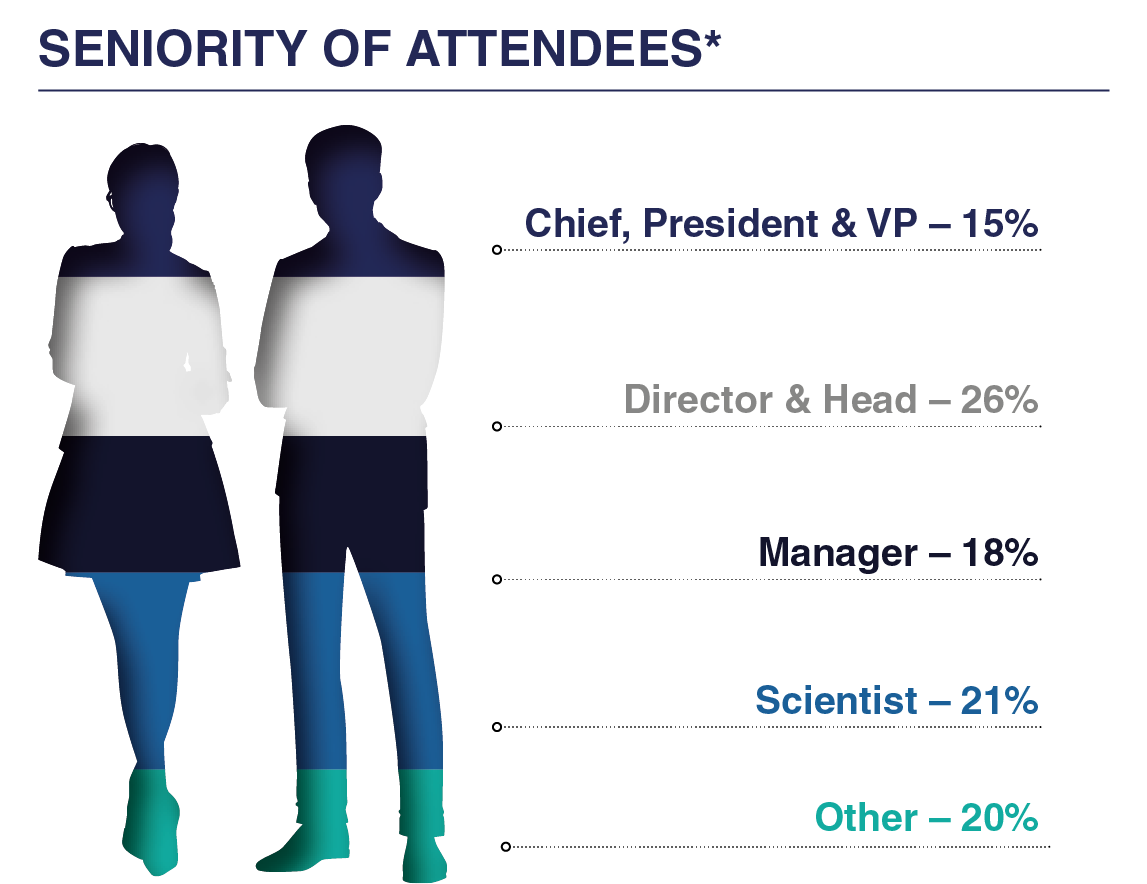
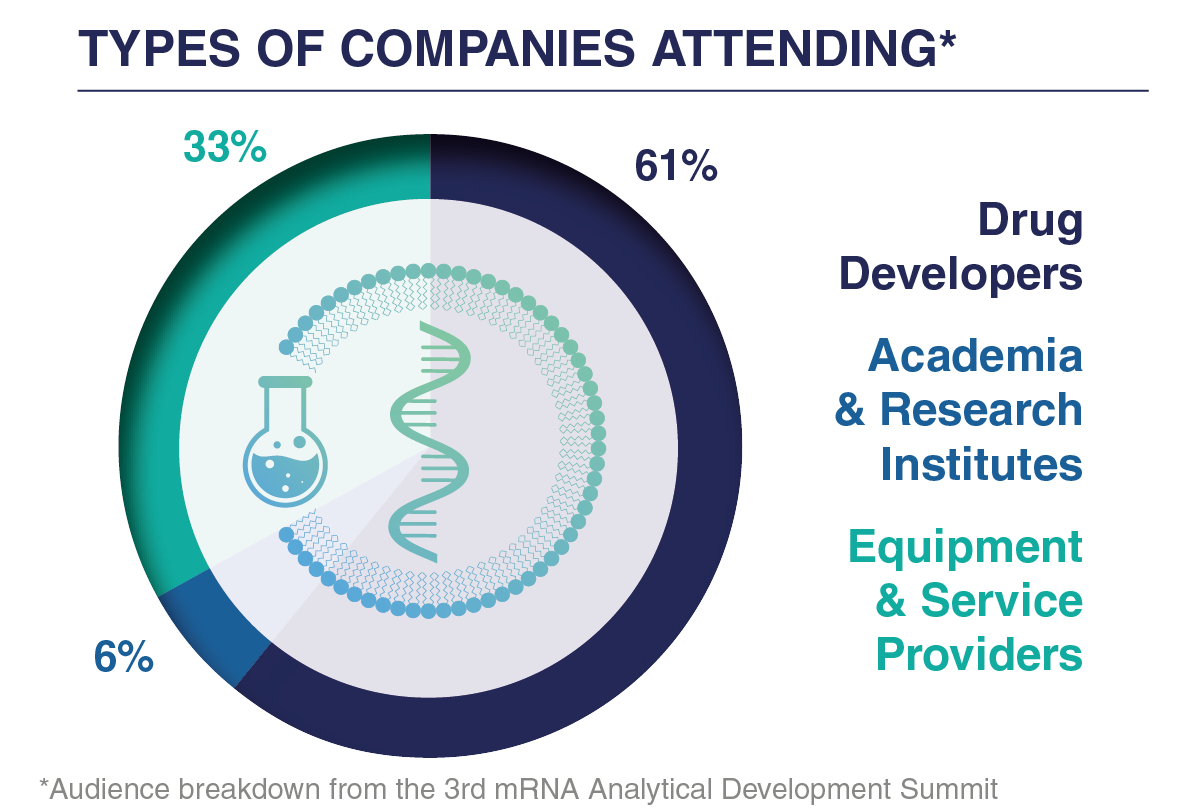
If you offer relevant services and would have liked to showcase your company to this rapidly evolving mRNA medicine and vaccine space, contact us to discuss a bespoke commercial package at the 2026 event.
For more information, email sponsor@hansonwade.com or download the full event guide.

